Gastrex
French excellence in the service of digestive health, combining innovation, reliability and certified quality.
Innovation at the heart of development
GASTREX focused on innovation early on by introducing Pronto Dry to the French market, a rapid urease test that allows the detection of Helicobacter pylori in a few minutes, directly during an endoscopy. This so-called "dry" test requires neither liquid solution nor refrigeration, making it a particularly popular solution in endoscopy rooms for its simplicity and speed.

Quality commitment and regulatory compliance
GASTREX manufactures its products in strict compliance with current regulatory requirements, ensuring rigorous control of each production stage. Our medical devices are developed in clean rooms, guaranteeing a controlled, clean environment that complies with the strictest standards to ensure the safety and optimal quality of products intended for healthcare professionals.
Innovation at the heart of development
GASTREX focused on innovation early on by introducing Pronto Dry to the French market, a rapid urease test that allows the detection of Helicobacter pylori in a few minutes, directly during an endoscopy. This so-called "dry" test requires neither liquid solution nor refrigeration, making it a particularly popular solution in endoscopy rooms for its simplicity and speed.
Quality commitment and regulatory compliance
GASTREX manufactures its products in strict compliance with current regulatory requirements, ensuring rigorous control of each production stage. Our medical devices are developed in clean rooms, guaranteeing a controlled, clean environment that complies with the strictest standards to ensure the safety and optimal quality of products intended for healthcare professionals.


In a constantly evolving medical world, GASTREX continues to adapt, innovate and offer concrete solutions, always in close collaboration with the field.
Our certificates
Discover our certificates

ISO13485:2016 Certificate

ISO13485:2016 Certificate
A company on a human scale, close to its customers
Location
Today, the GASTREX company is located in Brignais , a commune in the Rhône department (69) , in the Auvergne-Rhône-Alpes region. It has been managed by Medical Instruments Corporation France (MIC France) since March 29, 2023.
Implementation
Its head office is located at 7 chemin de Sacuny, 69530 Brignais . This location places it close to Lyon, thus facilitating its access to the region's transport networks and economic infrastructure.
Easy to use
While developing, GASTREX has therefore managed to maintain an agile and responsive structure , with customer service based in France , rapid logistics (same-day dispatch for all orders placed before 3 p.m.) and a strong commitment to the safety of care .


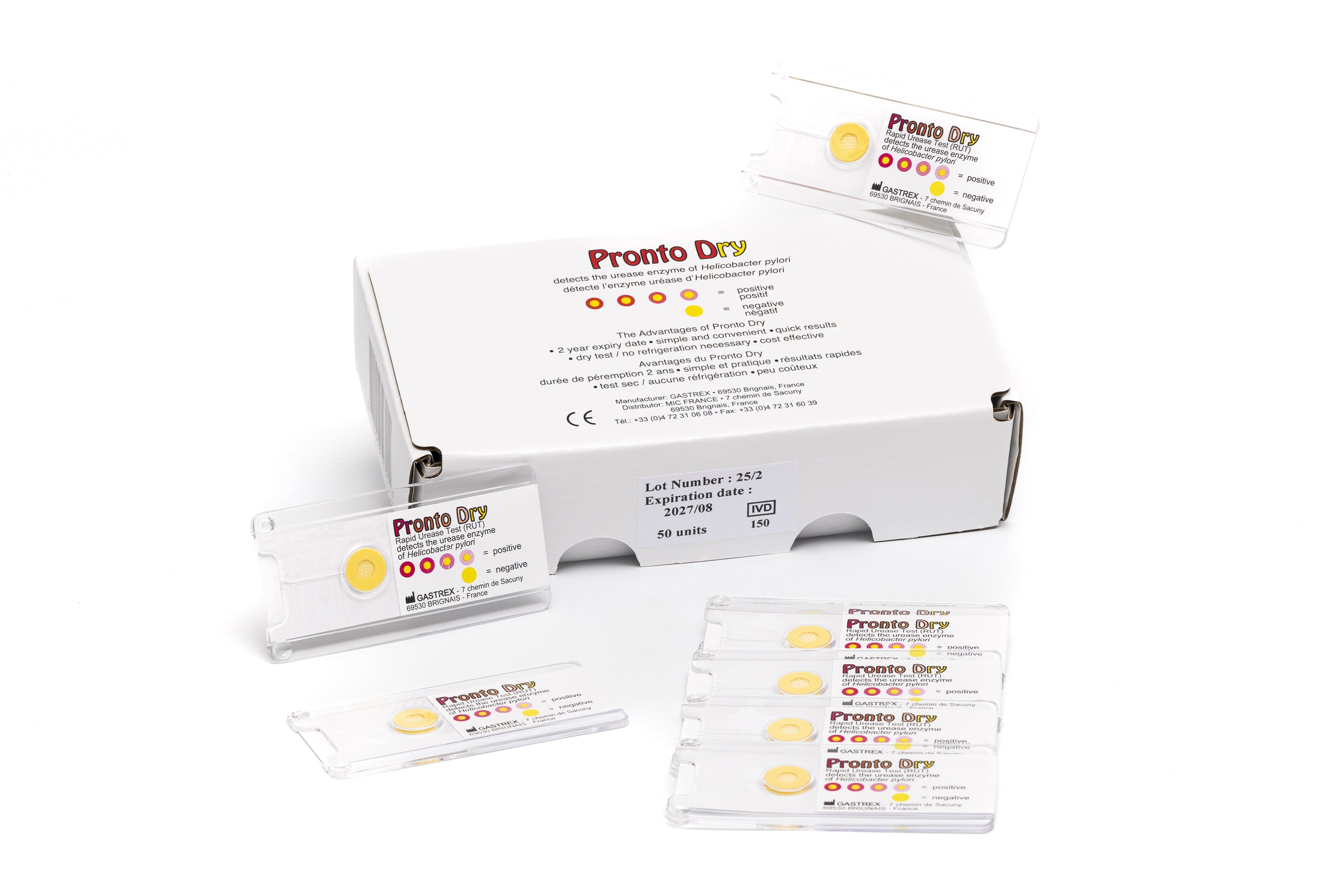
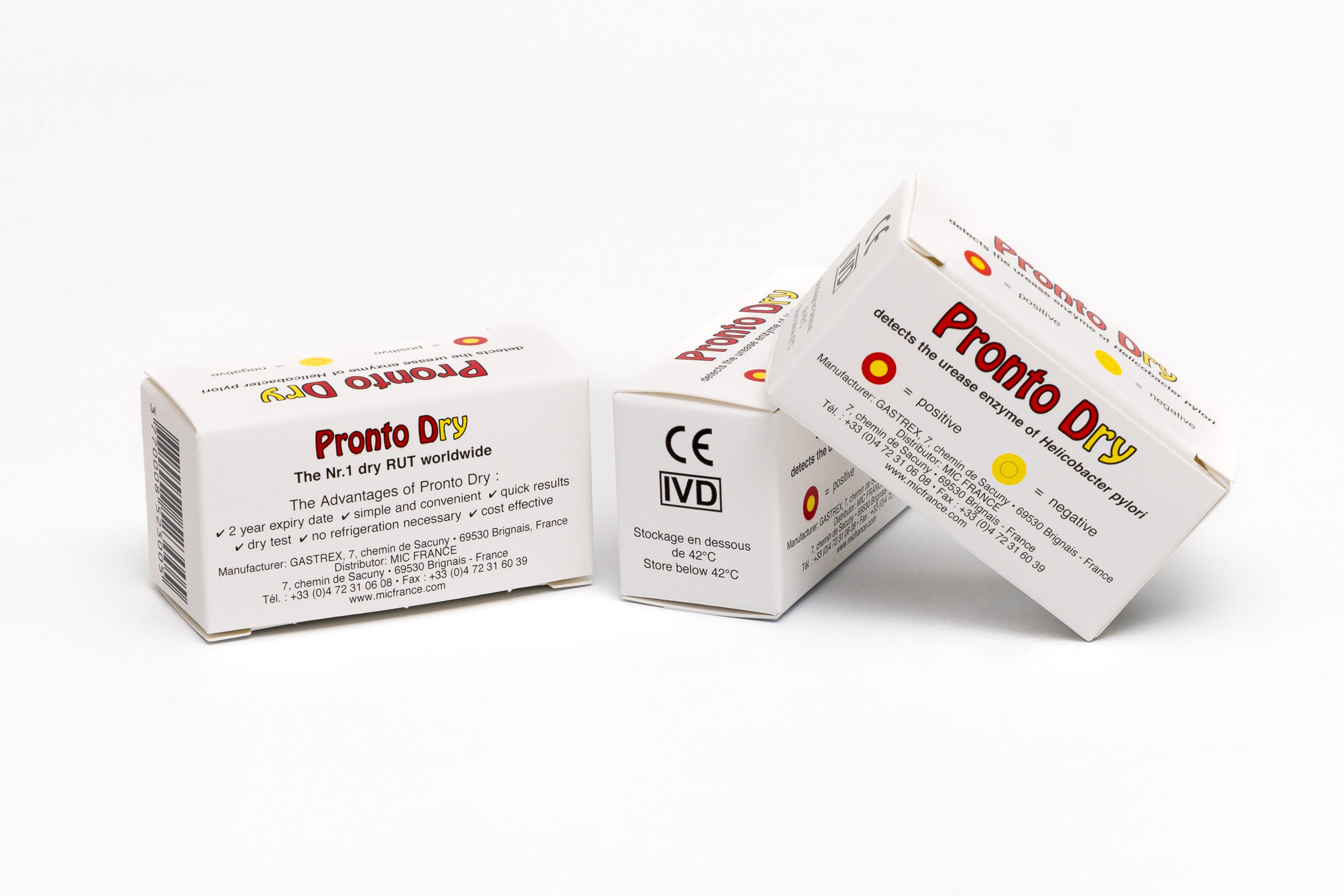
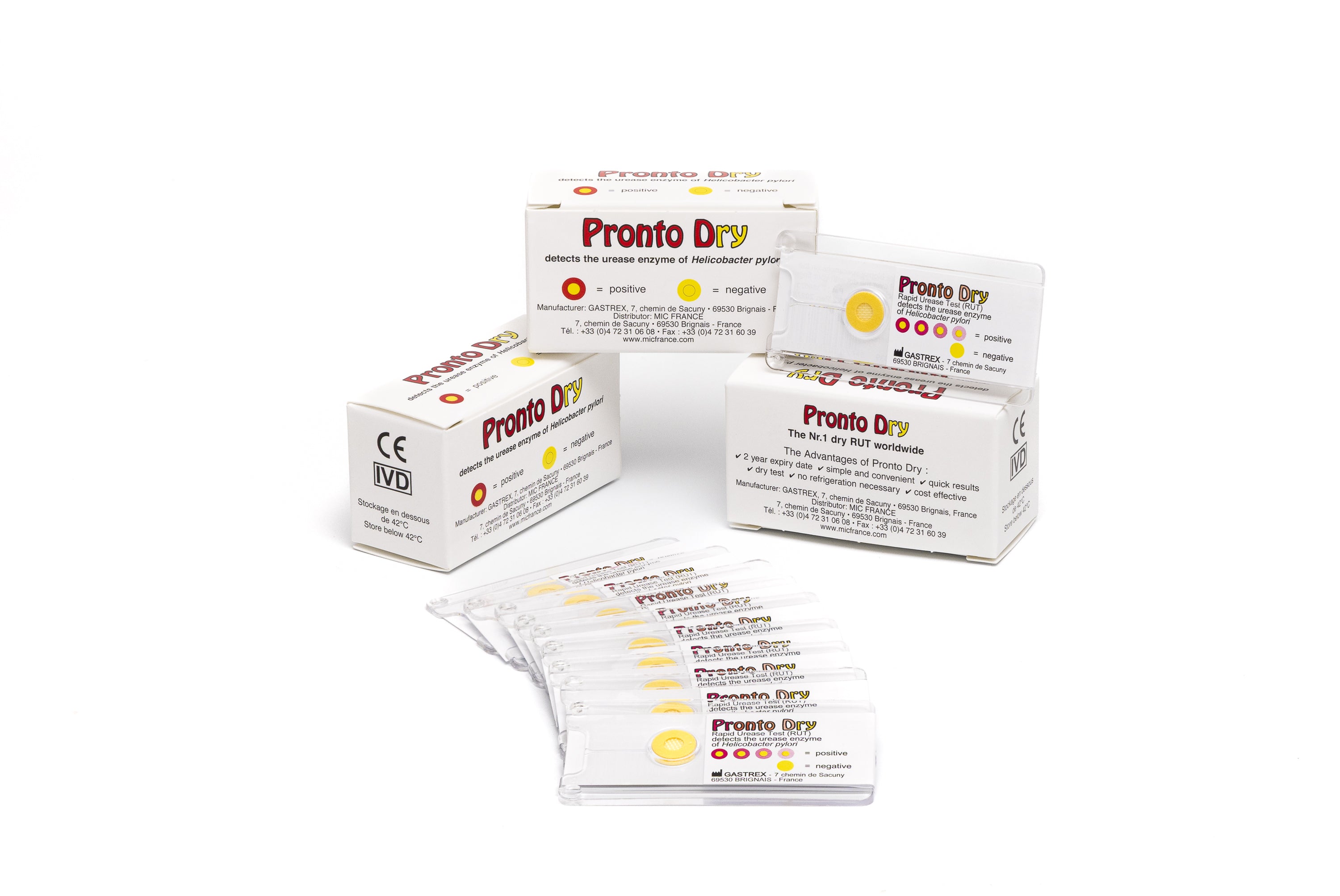
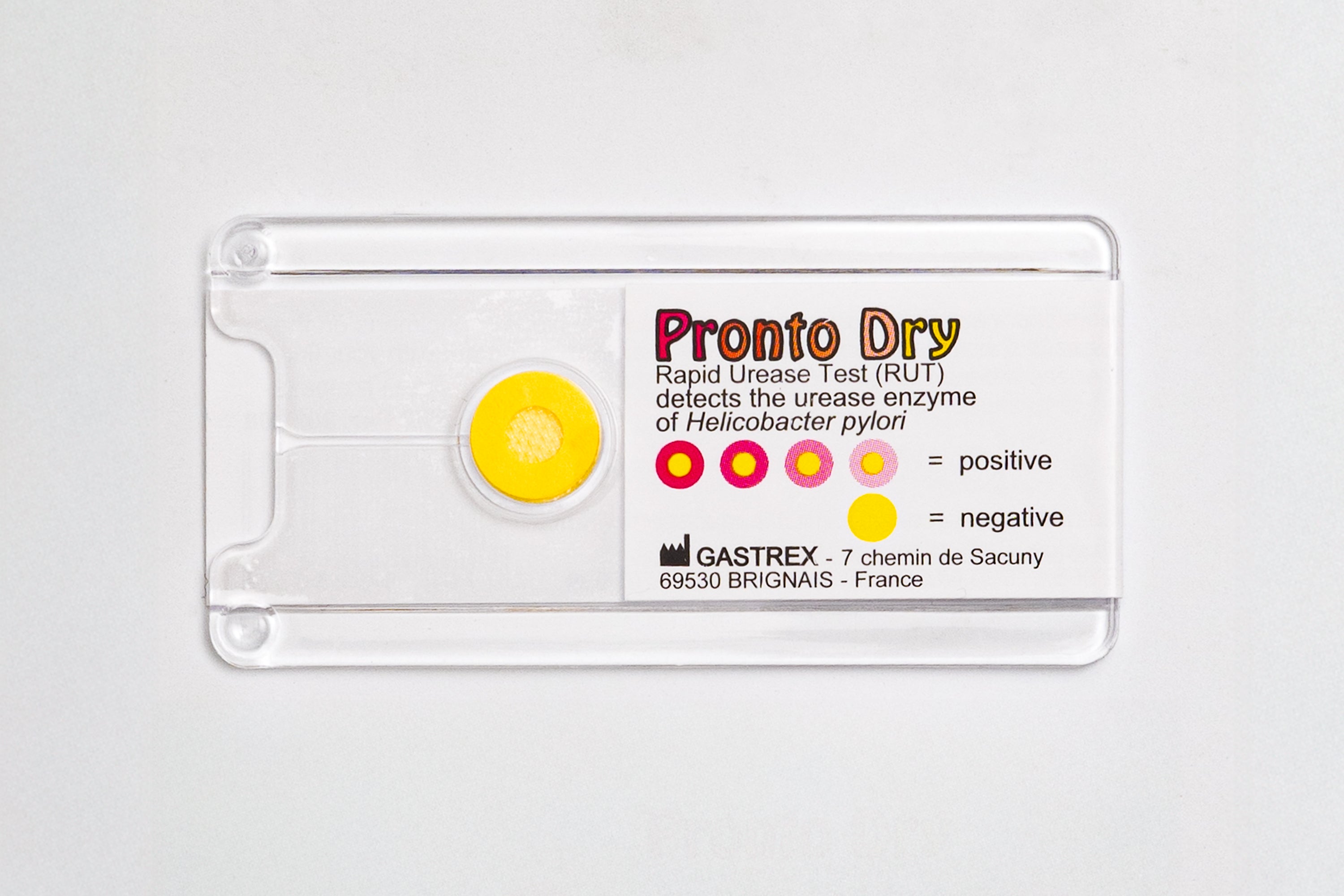
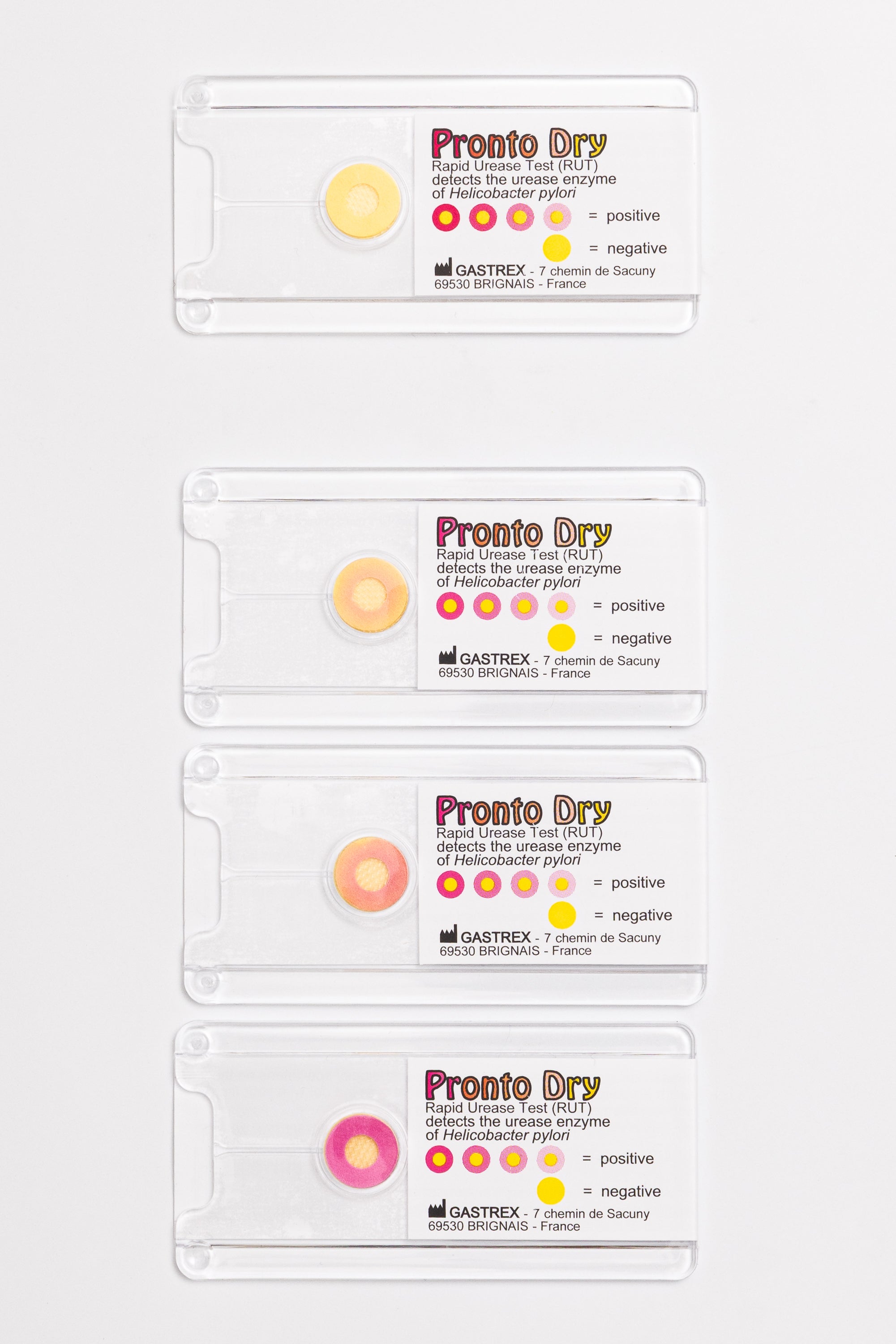

The Pronto Dry is a Rapid Urease Test (RUT) developed by Gastrex. It detects the urease enzyme in gastric mucosal biopsies in cases of suspected Helicobacter pylori in a symptomatic patient.